
[ad_2]
SAN FRANCISCO, Feb. 7, 2023 /PRNewswire/ — COLOTECT 3.0, a multi-target fecal DNA test outperforms fecal immunochemical tests (FIT) in terms of detection of colorectal cancer and advanced precancerous lesions.
BGI Genomics, a global life sciences company providing integrated solutions of precision medicine, presented case-control study data on its COLOTECT 3.0 non-invasive colorectal cancer screening test at the 2023 ASCO Gastrointestinal Cancers Symposium (ASCO GI 2023) and announced plans for further global clinical studies.
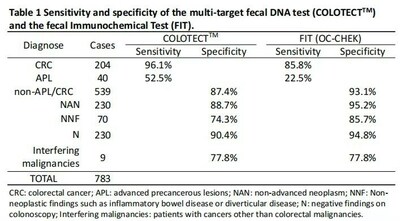
According to data released during ASCO GI 2023, in a recently concluded multi-center case-control study, BGI Genomics collected stool specimens from a total of 783 subjects with Colorectal cancer (CRC), advanced precancerous lesions (APL), non-advanced neoplasms and individuals with negative findings on colonoscopy from six sites across China. All participants underwent colonoscopy. Stool specimens from these participants were tested with the COLOTECT 3.0 multi-target fecal DNA test as well as a quantitative Fecal immunochemical test (FIT) (OC FIT-CHEK, Eiken) in parallel to perform head-to-head comparison of the test performance of both assays.
COLOTECT 3.0, a multi-target fecal DNA test demonstrated higher sensitivity (96.1%) than FIT (85.8%) for CRCs (n=204). Meanwhile, 52.5% of patients (n=40) with APL tested positive by COLOTECT 3.0, which was remarkably superior to FIT (22.5%). Among 539 participants with non-advanced neoplasms or negative findings on colonoscopy, the specificity of COLOTECT 3.0 was 87.4%, compared with a specificity of 93.1% achieved by FIT.
COLOTECT 3.0 is a novel multi-target fecal DNA based assay, which integrates detection of two stool DNA (sDNA) methylation markers by quantitative methylation specific PCR (qMSP) and immunochemical fecal occult blood test.
COLOTECT 3.0 has obtained CE certification and MHRA approval. For product details and availability, please contact info@bgi.com.
Contact
Fang Zhang
info@bgi.com
Image Attachments Links:
Link: http://asianetnews.net/view-attachment?attach-id=437693
Caption: ASCO GI 2023 Sensitivity and specificity of the multi-target fecal DNA test of COLOTECT 3.0 poster
Photo – https://mma.prnewswire.com/media/1996257/Poster.jpg
View original content:https://www.prnewswire.co.uk/news-releases/bgi-colotect-3-0-demonstrates-higher-colorectal-cancer-detection-sensitivity-than-fit-301740210.html
Source link
[ad_2]
The content is by PR Newswire. Headlines of Today Media is not responsible for the content provided or any links related to this content. Headlines of Today Media is not responsible for the correctness, topicality or the quality of the content.
[ad_2]

